KeifeRx’s Kinase Inhibitor technologies are designed to leverage mechanisms of action inherent to Kinase Inhibitors which thus far have been underexplored. This includes the ability to penetrate the brain, induce autophagy, and enable the bulk disposal of disease-causing toxic proteins to treat neurodegenerative diseases, and the ability to target mast cells and simultaneously modulate peripheral and central immunity, providing therapeutic potential for an array of immune diseases.
Compelling preclinical and clinical research conducted at Georgetown University by KeifeRx’s co-founder, Charbel Moussa, MBBS, Ph.D., has demonstrated the ability of Kinase Inhibitors to enable protein clearance and eliminate the inflammatory response. These properties offer the potential to significantly improve upon current neurodegenerative and immune disease treatments, which are primarily palliative and offer minimal and temporary benefits due to their inability to adequately eliminate toxic proteins and mitigate inflammation.
Explore KeifeRx’s Kinase Inhibitors technologies:
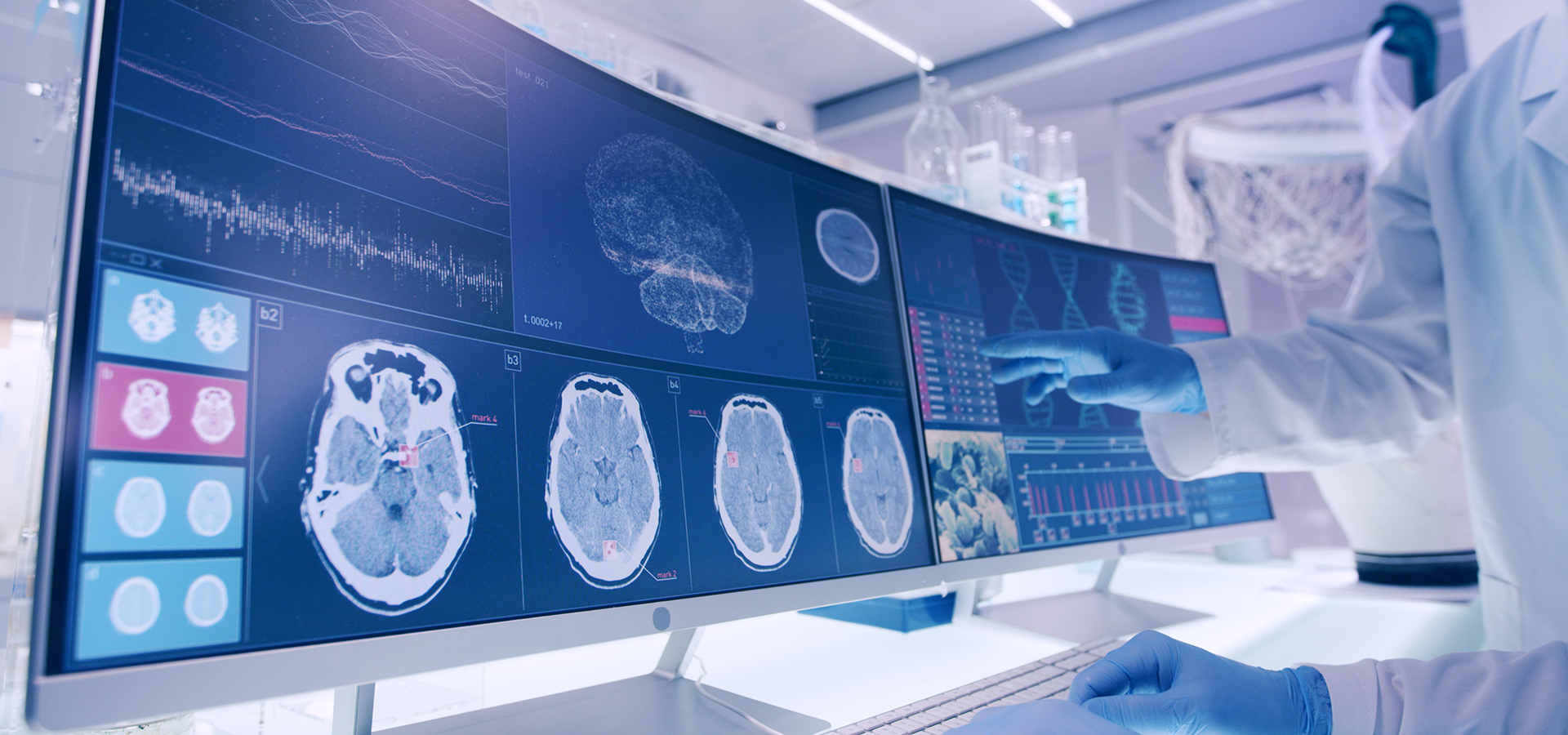
Kinase Inhibitors as a treatment option in neurodegeneration is a novel and strategic approach to triggering autophagy to clear neurotoxic proteins, reduce inflammation, and insulate irreplaceable brain cells via blood-brain-barrier protection.
Kinase Inhibitors have traditionally been used in cancer treatment. However, recent overwhelmingly strong evidence indicates that Kinase Inhibitors induce multiple changes that lead to mitigation of the toxic effects of misfolded proteins that are hallmarks of neurodegenerative diseases.
Kinase Inhibitors are known to inhibit specific receptors such as discoidin domain receptor (DDR)-1, Abl/Src and c-KIT to trigger autophagy, thus reducing toxic proteins and eliminating inflammation in the brain. Autophagy is a mechanism for maintaining cellular homeostasis whereby the cell collects and degrades unnecessary or dysfunctional components.
In the treatment of cancer, Kinase Inhibitors accelerate the clearance of unwanted molecules and mitigate faulty signaling mechanisms to suffocate tumors in mitotic cancer cells. Similarly, autophagy can degrade misfolded proteins and reduce accumulation of neurotoxic protein aggregates and halt inflammation in irreplaceable brain cells. Accumulation of neurotoxic proteins also leads to oxidative damage and stress that drains cellular energy, leading to neuronal death.
Additionally, recent data revealed abnormal levels of c-KIT in the postmortem brain and spinal cord of Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic Lateral Sclerosis (ALS) patients. As such, c-KIT inhibition offers the potential to regulate central and peripheral mechanisms of neuro-inflammation and neurotoxic protein clearance.
Collectively, the mechanisms of action of Kinase Inhibitors provide protection against multiple insults that cause neurodegenerative diseases, including AD, PD, Lewy Body Dementia, Huntington’s Disease, ALS, Multiple Sclerosis and many others.
Mast cells are hematopoietic stem cell-derived effector cells that function as part of the innate immune system at sites of injury. While mainly active in the periphery, they are also resident in the brain and play a role in initiating the neuro-inflammatory response.
Mast cells have been shown to promote a pro-inflammatory phenotype via activation of microglia, resulting in the release of inflammatory molecules. Additionally, microglia express histamine receptors, making them particularly receptive to mast cell activation and inflammatory signaling. Mast cells can also directly stimulate neuro-inflammation through release of inflammatory mediators such as tumor necrosis factor (TNF)-α and histamine.
Mast cells express c-KIT, a Kinase Inhibitors that regulate autophagy. c-KIT signaling on mast cells also regulates pathways involved in the mast cell inflammatory response. c-KIT has been shown to be expressed on both neurons and microglia, and its role in regulating the immune response to react to insult is a novel therapeutic option.
Inhibiting c-KIT may provide a means of both stimulating neurotoxic protein clearance via autophagy and attenuating mast cell-induced neuro-inflammation. Therefore, development of c-KIT inhibitors to enhance clearance via neuronal autophagy and reduce inflammation through mast-cell-glial interactions is a profoundly strong strategy to target multiple pathologies.
PUBLICATIONS
CSF MicroRNAs Reveal Impairment of Angiogenesis and Autophagy in Parkinson Disease
VIEW PUBLICATION
Long-Term Safety and Clinical Effects of Nilotinib in Parkinson’s Disease
VIEW PUBLICATION
Nilotinib Effects on Safety, Tolerability, and Biomarkers in Alzheimer’s Disease
VIEW PUBLICATION


